Introduction
Molecular minimal residual disease (MRD) has been shown to correlate with disease relapse in patients (pts) with Diffuse Large B cell Lymphoma (DLBCL)[1-4]. However, the clinical impact of detectable MRD (dMRD) prior to clinical relapse is unknown.
Checkpoint inhibitor (CPI) therapy has some activity in certain pts with DLBCL, particularly after CAR-T therapy attributed to effects on T cell activity [5-8]. We hypothesized that early intervention with CPI may lower relapse rate in pts with molecular recurrence only. Patients were monitored for detectable MRD (dMRD) by circulating tumor DNA (ctDNA) using the clonoSEQ assay monthly. We conducted a pilot study in high risk pts in remission after most recent line of therapy (LOT) in which maintenance nivolumab (MN) was offered at time of dMRD to assess effect on MRD clearance and relapse rate.
Methods
Pts >18 years with high-risk DLBCL (ABC-subtype, high grade B cell lymphoma (HGBCL), MYC translocation, relapsed/refractory disease, or Ki67 >90%) who had achieved CR on most recent LOT. Pts had monthly MRD and every 4 month CT or PET/CT and exam for 2 years. If conversion to dMRD without clinical relapse, pts received MN (240 mg IV q2 weeks) until clinical relapse or toxicity for up to 2 years (26 cycles). Pts with overt relapse at time of dMRD were ineligible for MN and taken off trial. The primary endpoint was rate of conversion from +MRD to undetectable MRD (uMRD) in pts receiving .
Results
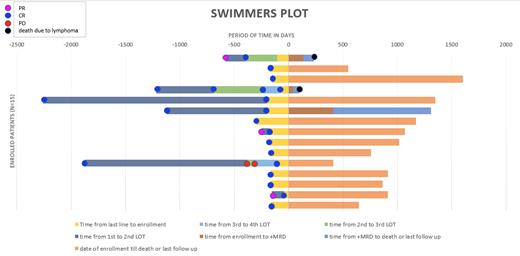
20 pts were screened and 15 pts were enrolled between June 2018 and March 2022. 5 pts had non-GCB subtype, 2 had GCB subtype, and two had unknown cell of origin. 4 had transformed DLBCL (2 from FL, 1 from MZL, and 1 Richter's), 2 pts had HGBCL (one of which was also transformed). Of the 8 pts who enrolled after 1 prior LOT, none had molecular or clinical relapse (Figure). In 7 pts who enrolled after 2+ prior LOT, 3 had dMRD. 2/3 had clinical relapse at same time as dMRD and were taken off study– one at initial enrollment with dMRD at first ctDNA screening and one converting from uMRD to dMRD after 135 days post enrollment. In the single patient who was eligible for MN, dMRD preceded radiographic relapse by 105 days, 410 days after enrollment. The pt went on to receive 2 cycles of MN before clinical progression and was taken off study. This pt failed to achieve conversion of dMRD to uMRD.
Conclusions
Due to small sample size and lower than expected relapse rates, we were unable to demonstrate conversion of dMRD to uMRD using MN. Of the 3 pts with dMRD, 2 were not eligible for MN due to clinical relapse at same time as dMRD and both died due to lymphoma progression. In the single pt who received MN, this intervention did not achieve uMRD or prevent relapse. However, this pt went on to achieve CR to subsequent CAR-T and remains in prolonged remission despite multiple relapsed/refractory disease. It is unknown if this patient's positive outcome was impacted by the effect of nivolumab on T cells and/or related to slower disease kinetics as this pt had a 3 month period between dMRD and radiographic detection and long duration of response to prior lines. A larger study is needed to adequately assess impact of early intervention MN using MRD kinetics, particularly in patients receiving CAR-T.
All pts with dMRD had radiographic relapse within 4 months of molecular relapse. No pts with uMRD by ctDNA had clinical relapse on study during 2 year follow up, confirming uMRD correlates with disease free survival. If a strong negative predictive value is confirmed in a larger study, ctDNA MRD testing may be used to spare pts from imaging or to guide additional work up in pts with inconclusive findings on scans.
OffLabel Disclosure:
Nakhoda:BTG/SERB: Consultancy, Honoraria; ADC Pharmaceuticals: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Matasar:Merck: Current equity holder in private company; Seagen: Honoraria, Other: stipends; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Other: Stipend; ADC Therapeutics: Consultancy, Honoraria, Other: Stipend; Teva: Consultancy; Janssen: Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Immunovaccine Technologies: Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Research Funding; Juno: Consultancy; Regeneron: Honoraria, Other: Stipends; Kite: Honoraria, Other: Stipends; Celegene: Honoraria, Other: Stipends; Epizyme: Other: Stipends; Bayer: Consultancy, Honoraria, Research Funding; Immunovaccine Technologies: Honoraria; BMS: Honoraria, Other: Stipend. Frosch:Seagen: Membership on an entity's Board of Directors or advisory committees; AstraZenica: Research Funding; Abbvie: Research Funding; Roche: Research Funding. Tan:Takeda: Research Funding; Janssen: Research Funding. Jacob:Adaptive Biotechnologies: Current Employment, Current equity holder in publicly-traded company.
Nivolumab as a maintenance therapy in patients with detectable minimal residual disease prior to radiographic relapse

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal